|
500 BC |
The Solid, Indivisible Sphere
|
|||||||||
|
|
Alchemists - searched for the Philosopher's Stone, which had the ability to transform base materials like copper or lead, into valuable substances, like gold. They also searched for the Elixir of Life, which when drunk by a particular person, would grant him immortality. | |||||||||
|
|
450 BC |
|||||||||

|
Democritus, Greece - stated that all matter is made up of atoms. He also stated that atoms are eternal and invisible and so small that they can’t be divided, and they entirely fill up the space they’re in. |
400 BC |
||||||||
|
|
Aristotle, Greece - provided the method of gathering scientific facts, which proved as the basis for all scientific work. |
|||||||||
|
350 BC |
||||||||||
|
Model II Planetary System |
||||||||||
|
skip to |
1700's |
|||||||||
| 1750 AD | ||||||||||
 |
Lavoisier (1777), France - provided the formula for the conservation of matter in chemical reactions, and also distinguished between an element and a compound. |
|
||||||||
|
Model III The Plum-Pudding |
||||||||||
|
|
|
Couloumb (1780's), France - formulated the Coulomb's law, which states that that the force between two electrical charges is proportional to the product of the charges and inversely proportional to the square of the distance between them, one of the main forces involved in atomic reactions. | ||||||||
|
1800 AD |
||||||||||
|
|
John Dalton (1803), England - formed the atomic theory, which states that all matter is composed of tiny, indestructible particles called atoms that are all alike and have the same atomic weight. | |||||||||
|
1850 AD |
||||||||||
|
|
Crookes (1870), England - created the Crookes’ tube and demonstrated that cathode rays travel in straight lines and produce phosphorescence and heat when they strike certain materials. | |||||||||
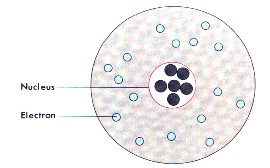
Model IV Rutherford's Nucleus Atom |
||||||||||
|
|
W.K. Roentgen
(1895), Germany - discovered
x-rays while experimenting with cathode-ray tubes.
|
|||||||||
|
|
Becquerel (1896), France - discovered radioactivity when he investigated uranium and other radioactive substances. |  |
The Curies (1898), France - discovered radium and polonium when they started to investigate radioactive substances |
|||||||
|
|
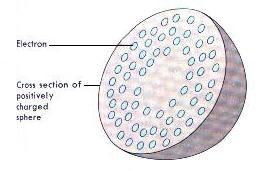
J.J Thomson (1898), England - discovered the electron and developed the plum-pudding model of the atom. |
|||||||||
|
1900 AD |
||||||||||
|
|
Max
Planck (1900), Germany -
originated the quantum theory
|
|
Albert Einstein (1905), Germany - postulated that light was made up of different particles that, in addition to wavelike behavior, demonstrate certain properties unique to particles. He also brought forth the theory of relativity. | |||||||
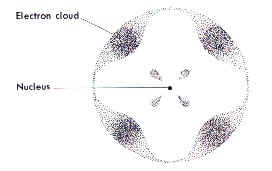
The Electron Cloud Atom |
||||||||||
 |
Robert Millikan (1908), USA - found out the electric charge of the electron | |||||||||
|
|
Ernest Rutherford (1909), England - used the results of his gold-foil experiment to state that all the mass of an atom were in a small positively-charged ball at the center of the atom. | |||||||||
|
|
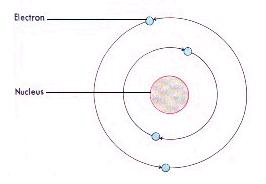
Neils Bohr (1913), Denmark - stated that the electrons moved around the nucleus in successively large orbits. He also presented the Bohr atomic model which stated that atoms absorb or emit radiation only when the electrons abruptly jump between allowed, or stationary, states. | |||||||||
|
|
Geiger (1925), Germany - introduced the first detector of alpha particles and other radiations. |
|
|
|||||||
 |
Erwin Shroedinger (1926), Austria - introduced the Shroedinger Equation, a wave equation that describes the form of the probability waves that govern the motion of small particles and how these waves are altered by external influences. | |||||||||
 |
Chadwick (1931), England - discovered the neutrally-charged neutron. | |||||||||
|
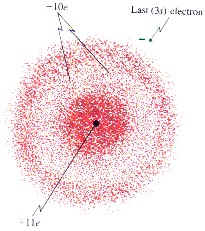
Detail of an Electron Cloud
|
||||||||||
 |
Otto Hahn (1938), Germany - discovered nuclear fission, in which the nucleus of an atom breaks up into two separate nuclei, while experimenting with uranium. |
|
 |
Lise Meitner (1938), Vienna - worked with Otto Hahn to discover uranium fission. | ||||||
| 1950 AD | ||||||||||
 |
Glen T. Seaborg (1951), USA - isolated and identified elements heavier than uranium, and in the process, added elements number 94 - 102, and 106. | |||||||||
 |
Murray Gell-Mann and George Zweig (1964), USA - brought forth the idea of "quarks", little bits of matter which when used kind of like building blocks, serve to explain some complex chemical substances. | |||||||||
| 2000 AD | ||||||||||